CT/NG/MG Multiplex PCR Kit
Manufacturer: 
Description
The PCR kit is designed for Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium detection by the real-time Polymerase Chain Reaction (PCR) method.
Technical Features
Specifications:
- Target Sequence: the cryptic plasmid sequence and the 16S rRNA gene for Chlamydia trachomatis/the 16S rRNA gene and porA pseudogene for Neisseria gonorrhoeae/the 16S rRNA gene for Mycoplasma genitalium
- Analytical Specificity: Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium, 100 %
- Analytical Sensitivity (LoD): reaches up to 0.177 cp/µl with the probability of 95 % (on Amplirun® Chlamydia trachomatis DNA control) reaches up to 0.22 cp/µl with the probability of 95 % (on Amplirun® Neisseria gonorrhoeae DNAcontrol), up to 1.129 cp/µl with the probability of 95 % (on Amplirun® Mycoplasma genitalium DNAcontrol) and up to 1.129 cp/µl with the probability of 95 % (on Amplirun® Mycoplasma genitalium DNAcontrol)
- Diagnostic Sensitivity: 97.67%
- Diagnostic Specificity: 96.89%
- Quality management system is certified in compliance with the requirements of the standard ISO 13485 ed.2:2016
- External Quality Assessment: regularly tested by QCMD and Instand e.V. External Quality Assessment Panels
- Required Detection Channels FAM + HEX + Cy5 + TexRed
- Compatible with a wide range of Real-time PCR devices (CFX96™/Dx Real-Time PCR Detection System, Applied Biosystems 7300 / 7500 Real-Time PCR System, Rotor-Gene 3000 /Q κα)
- Stable at -20 ± 5 °C
- The components are stable for a maximum of 3 repeated freezing / thawing cycles
- Regulatory Status CE₁₀₂₃ IVD
Format
- 25/50/100 reactions
Shelf life
- 18 months from production date
Sample
- Swab, urine
Test time
- 65 min
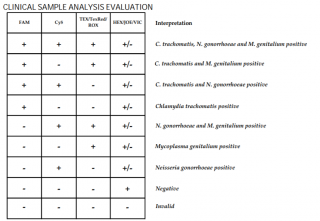
Results