Influenza A, B and RSV multiplex PCR kit
Manufacturer: 
Description
The PCR kit is intended for detection of Influenza A, Influenza B and Respiratory Syncytial Virus (RSV) A/B RNA by the reversetranscription real-time Polymerase Chain Reaction (PCR) method.
Technical Features
Specifications:
- Target Sequence: specific region in M gene and NP gene for Influenza A virus/two specific regions in HA gene for Influenza B virus/specific region in M gene for Respiratory syncytial virus A and B
- Analytical Specificity: Influenza A virus, Influenza B virus and Respiratory syncytial virus A and B, 100 %
- Analytical Sensitivity (LoD): reaches up to 161.64 cp/ml for Influenza A up to 7.25 cp/µl for Influenza B virus (on Amplirun® Influenza B RNA control)up to 43.57 cp/µl for Respiratory syncytial virus (on Amplirun® RSV A RNA control)
- Diagnostic Specificity 100.00% (CI95%: 79.95% – 100.00%)
- Diagnostic Sensitivity 92.31% (CI95%: 80.60% – 97.51%)
- Quality management system is certified in compliance with the requirements of the standard ISO 13485 ed.2:2016
- External Quality Assessment: regularly tested by QCMD and Instand e.V. External Quality Assessment Panels
- Required Detection Channels FAM+ HEX + TexRed + Cy5
- Compatible with a wide range of Real-time PCR devices (LineGene 9600 Plus, Applied Biosystems 7300 / 7500 Real-Time PCR System, CFX96™/ Dx Real-Time PCR Detection System κα)
- Stable at -20 ± 5 °C
- The components are stable for a maximum of 3 repeated freezing / thawing cycles
- Regulatory Status CE IVD
Format
- 25/50/100 reactions
Shelf life
- 18 months from production date
Sample
- Aspirate, BAL, Swab
Test time
- 75 min
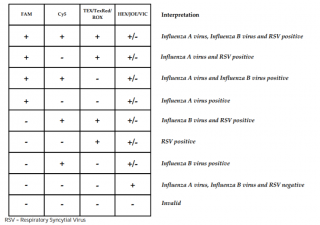
Results