Compass Listeria
Description
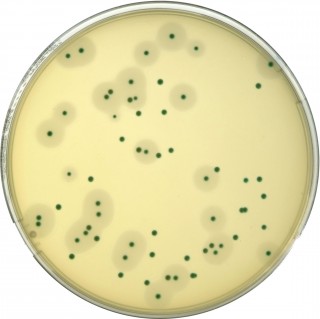 The COMPASS® Listeria method allows the detection and enumeration of Listeria monocytogenes and Listeria spp. in human food products and environmental samples.
The COMPASS® Listeria method allows the detection and enumeration of Listeria monocytogenes and Listeria spp. in human food products and environmental samples.
Technical specifications
Features
- Reliable Validated method by AFNOR Certification according to ISO 16140 standard and formulated to the NF EN ISO 11290-1/A1 and NF EN ISO 11290-2/A1 (Ottaviani Agosti Agar)
- Performance Absence of secondary enrichment and excellent selectivity of COMPASS® Listeria Agar for reading and optimal enumeration
- Simple and economic Easily adaptable to all laboratory organizations: – Use of Half-Fraser Broth for both detection and enumeration protocols – Enrichment and agar may be conserved for 3 days
- Quick Negative screening in 44 hours and confirmation of L. monocytogenes in only 6 hours
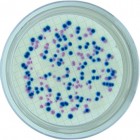
- Easy Clear distinction between BLUE colonies WITH halo of Listeria monocytogenes and BLUE colonies WITHOUT halo of Listeria spp.
Format
- 500gr. bottle, 20 & 120 plates and 6×200 ready to use vials
Sample kind
- Human food products and environmental samples
Test time
- 24 hours for detection or enumeration
Results
- Blue color with and / or without halo
Documents
Compass Listeria
Compass Listeria Agar Enumeration
Approvals / Certificates
Certification Compass Listeria