Boson nCov-2 Antigen Test
Manufacturer: ![]()
Description

The BOSON antigen test is one of the few in the world that has been licensed and marketed by the German (BfArM) and European (CIBG) drug organization and has been marketed in European pharmacies and supermarkets. It is also included in the list of recommended EU antigen tests (link: EU common list of Covid-19 rapid antigen tests)
This is a rapid immunochromatographic flow test for the rapid assay of SARS-CoV-2 virus antigen (nucleocapsid protein N protein) in nasopharyngeal smears from individuals suspected of having COVID-19
The sample must be handled – as in all laboratory samples – as a potential pathogen. Strictly for professional use in laboratories or coronavirus care points. No specialized staff or analyst is required to use it. Valid results in 15-20 ‘.
Technical Specifications
Features
- It has 99.22% specificity and 96.49% sensitivity
- Meets all the conditions of Government Gazette 5198 / 24.11.20-01.02.2021
- It is approved by the AEO of Germany BfARM
- It gives results in just 15 minutes
- It is on the FIND list It is on the WHO list for approval
- The kit is ready to use and contains all the equipment needed to perform the tests. Detects virus mutations
Format
- 20 tests
Sample
- Nasopharyngeal smear
Test time
- 15-20 min
Shelf life
- 18 months from production date
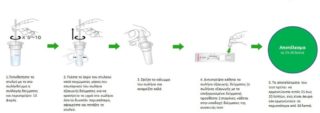
Procedure
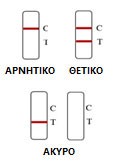
Result Interpretation
Documents
nCov2 Τεστ Αντιγόνου – Interpretation guide
Insert BS-SARS-CoV-2 Ag -Specificity 99,22% A00 210225
Clinical Evaluation Report – Specificity 99,222%-210225
Clinical Evaluation Report-201217 positive case 102 person (1)
Performance Characteristics Study of Boson SARS-CoV-2 Antigen Test UK (2)
MHRA Registration_Confirmation_Letter
Statement for Boson Antigen Test approval by German and Switzerland Govenment